Impurities >> Prednisolone / Loteprednol etabonate Impurities
![]() +91 81692 39822 / +91 99873 02910
+91 81692 39822 / +91 99873 02910
![]() pharmachem@karandikars.in / info@kpharmachem.com
pharmachem@karandikars.in / info@kpharmachem.com
Impurities >> Prednisolone / Loteprednol etabonate Impurities
| Product Name | CAS Number | Molecular Structure | Molecular Formula | Molecular Weight | IUPAC Name |
|---|---|---|---|---|---|
| Prednisolone | 50-24-8 | 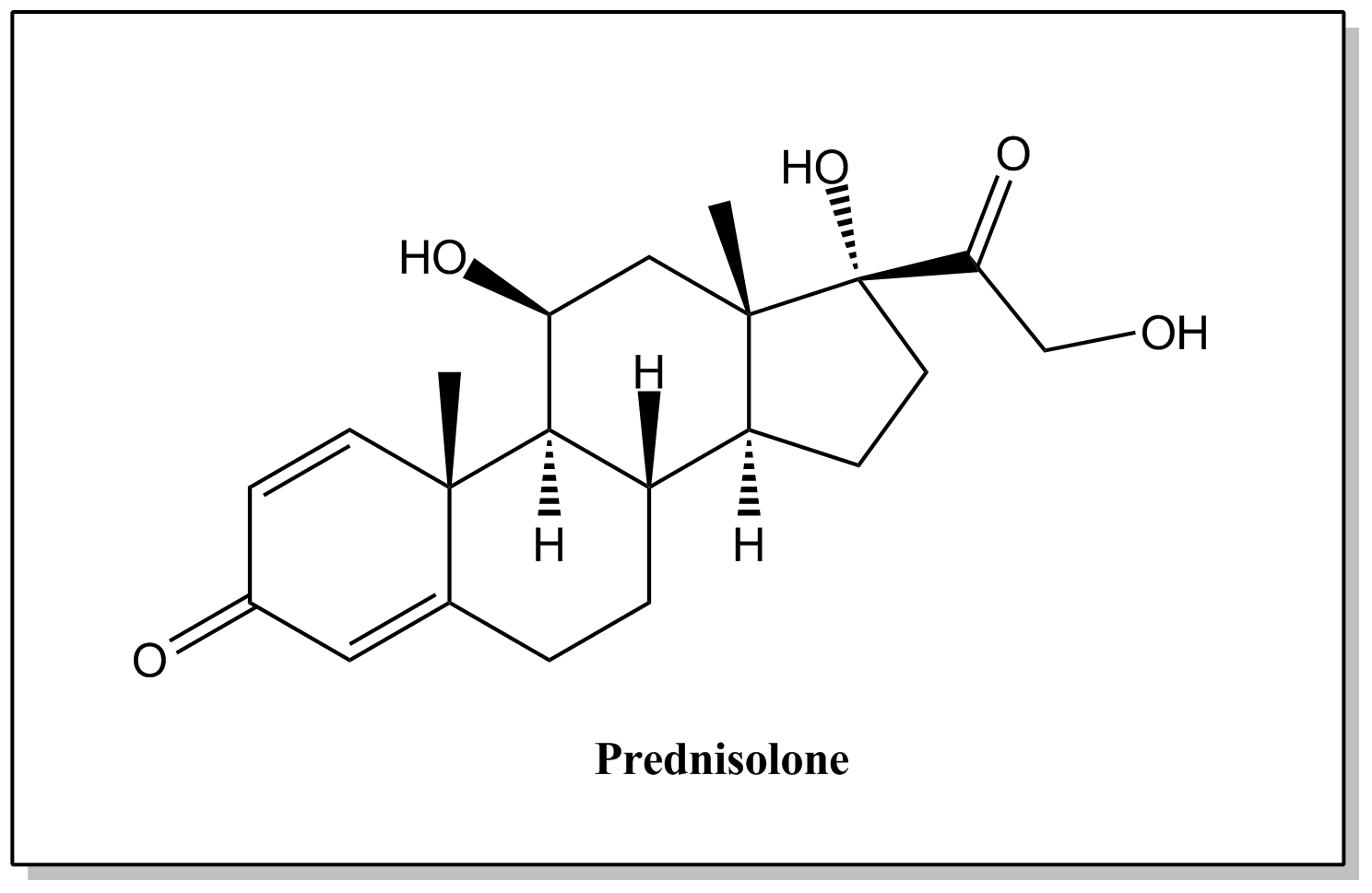 | C21H28O5 | 360.44 | (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthrene-3,11(6H)-dione |
| Prednienic Acid | 37927-29-0 | 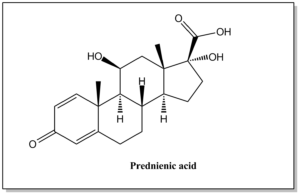 | C20H26O5 | 346.42 | (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthrene-17-carboxylic acid |
| Prednisolone 20-ethyl ester | N / A | 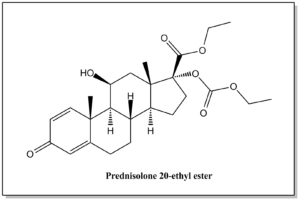 | C25H34O7 | 446.54 | (8S,9S,10R,11S,13S,14S,17R)-ethyl 17-((ethoxycarbonyl)oxy)-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthrene-17-carboxylate |
| Loteprednol etabonate methyl ester | N / A | 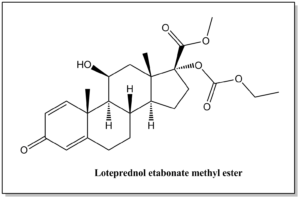 | C24H32O7 | 432.51 | (8S,9S,10R,11S,13S,14S,17R)-methyl 17-((ethoxycarbonyl)oxy)-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthrene-17-carboxylate |
| Prednisolone EP impurity B | 53-03-2 | 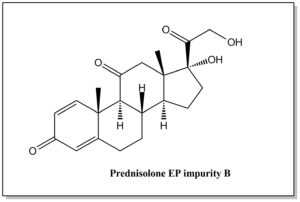 | C21H26O5 | 358.43 | (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthrene-3,11(6H)-dione |
| Prednisolone 17-(ethyl carbonate) 17-carboxylic acid | N / A | 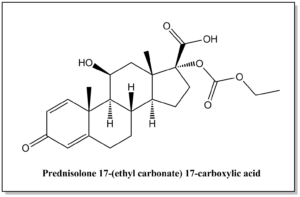 | C23H30O7 | 418.48 | (8S,9S,10R,11S,13S,14S,17R)-17-((ethoxycarbonyl)oxy)-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthrene-17-carboxylic acid |
Disclaimer
Products under patent are offered for R&D use only. These products cannot be offered to the countries where patent law is in force. Ultimate responsibility lies wholly with the buyer of our products.